I've watched folks shoot their 308 Win match rifles at Camp Perry's 575 foot elevation in 1000 yard matches with the same ammo and zeros used at the NRA Whittington center's 6600 foot level put their first shot off the 6 foot square target's bottom. Then do it several more times before realizing the thicker air slowed their bullets down enough to do that.
-
If you are being asked to change your password, and unsure how to do it, follow these instructions. Click here
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Altitude vs. Barometric pressure
- Thread starter Michael Eichele
- Start date
 Help Support Long Range Hunting Forum
Help Support Long Range Hunting Forum
Planes like low density altitude, bullets like high density altitude
User4302021
New Member
- Joined
- Mar 17, 2018
- Messages
- 0
I have a GDF-100. Several models of the ProTrek will do it also. The rep you talked to probably didn't have any idea what station pressure was. Any of the G-Shocks that have altitude sensors will show both altitude (effectively, pressure altitude), as well as the raw data of station pressure.Dog Rocket, which Casio model do you have? I called Casio to inquire about a model that gives SP and the rep could not tell me a model. That would give me a backup to my Weathermate Weather Hawk.
I've wanted a RISEMAN for quite sometime, but the reality is that this one does everything I need.
PApa Black
Well-Known Member
- Joined
- May 23, 2012
- Messages
- 460
I use this program: fill in all the blanks and the correct answer is calculated: https://www.jbmballistics.com/ballistics/calculators/calculators.shtmlExample: RSI Ballistic calculator calculates that at sea level in 59 degree air and 29.53 BP a bullet with a .540 BC at 2700 FPS zeroed at 300 yards will drop 307.9" at 1000 yards.
The same program, same BP, bullet, velocity, zero, temprature, but change the altitude to 5000' and the bullet drop is calculated to be 315.8 with the same barometric pressure imput.
The differance is 7.9"
I always thought that the actual pressure and not altitude changed the bullets perfomance. I know pressure changes with higher/lower altitudes go hand in hand, but thought that altitude in and of itself had no effect on the bullet and just the pressure change with the altitude.
Any ideas?? Is the RSI program correct or is it a glitch?
Mikecr
Well-Known Member
Mram did not suggest that low density altitude is thin air. It isn't.
YOUR confusion provides another example of why shooters need to steer clear of DA for solutions.
YOUR confusion provides another example of why shooters need to steer clear of DA for solutions.
Well then, what is low density altitude? What has less per cubic unit?Mram did not suggest that low density altitude is thin air. It isn't.
Mikecr
Well-Known Member
Last edited:
Thanks for the link.
Sorry I made you upset about my query that you chose to "internet shout" using all caps addressing me.
Hugnot
Well-Known Member
In the old days, we were issued barometer/altimeters for making elevation determinations. The deal was to calibrate the altimeter at a known elevation then use change in pressure for elevation determinations. Now GPS is available and ends the problem when storms roll in. Ground mapping is much better now. The common Miller twist/stability program uses barometric pressure and temperature.
Mikecr
Well-Known Member
We're not making elevation determinations, but air density determinations.
They would be opposite if not so disconnected.
They would be opposite if not so disconnected.
Hugnot
Well-Known Member
When I ascend from 3000 feet to 7500 feet I would expect the air density to decrease and my GPS would provide an elevation but as the temperature changed or if a storm rolled in or out with moisture and barometric pressure changes the air density would change. Various bullet stability calculations, the most common being the Miller program use barometric pressure and temperature to estimate Sg values.
ρ = mass of air / volume
The standard measurement type for air density is kilograms per cubic meter (Kg/m3). Many people also use the following:
g/cm³ = gram per cubic centimeter
1 g/cm³ = 0.001 kg / m³
kg/L = kilogram per liter
1kg/L = 1,000 kg / m³
g/mL = gram per milliliter
1g/mL = 1,000 kg / m³
Air density decrease with altitude increase.
The first reason is gravity. Earth's gravity pulls air as close to the surface as possible. The second reason is density. As altitude increases, the amount of gas molecules in the air decreases—the air becomes less dense than air nearer to sea level.
The amount of water vapor in the air also affects the density. Water vapor is a relatively light gas when compared to diatomic Oxygen and diatomic Nitrogen. Thus, when water vapor increases, the amount of Oxygen and Nitrogen decrease per unit volume and thus density decreases because mass is decreasing.
ρ = mass of air / volume
| Altitude [ft (m)] | Temperature [°F (°C)] | Pressure [psi (hPa)] | Air density [lb / cu ft (kg / m³)] |
|---|---|---|---|
| sea level | 59 (15) | 14.7 (1013.25) | 0.077 (1.23) |
| 2 000 (610) | 51.9 (11.1) | 13.7 (941.7) | 0.072 (1.16) |
| 4 000 (1219) | 44.7 (7.1) | 12.7 (873.3) | 0.068 (1.09) |
| 6 000 (1829) | 37.6 (3.1) | 11.7 (808.2) | 0.064 (1.02) |
| 8 000 (2438) | 30.5 (-0.8) | 10.8 (746.2) | 0.06 (0.95) |
| 10 000 (3048) | 23.3 (-4.8) | 10 (687.3) | 0.056 (0.9) |
| 12 000 (3658) | 16.2 (-8.8) | 9.2 (631.6) | 0.052 (0.84) |
| 14 000 (4267) | 9.1 (-12.8) | 8.4 (579) | 0.048 (0.77) |
| 16 000 (4877) | 1.9 (-16.7) | 7.7 (530.9) | 0.045 (0.72) |
The standard measurement type for air density is kilograms per cubic meter (Kg/m3). Many people also use the following:
g/cm³ = gram per cubic centimeter
1 g/cm³ = 0.001 kg / m³
kg/L = kilogram per liter
1kg/L = 1,000 kg / m³
g/mL = gram per milliliter
1g/mL = 1,000 kg / m³
Air density decrease with altitude increase.
The first reason is gravity. Earth's gravity pulls air as close to the surface as possible. The second reason is density. As altitude increases, the amount of gas molecules in the air decreases—the air becomes less dense than air nearer to sea level.
The amount of water vapor in the air also affects the density. Water vapor is a relatively light gas when compared to diatomic Oxygen and diatomic Nitrogen. Thus, when water vapor increases, the amount of Oxygen and Nitrogen decrease per unit volume and thus density decreases because mass is decreasing.
Last edited:
Just to add my CHF 0.02 to the old thread. In case you were wondering how pressure measurements affect hit probability, here's a picture:
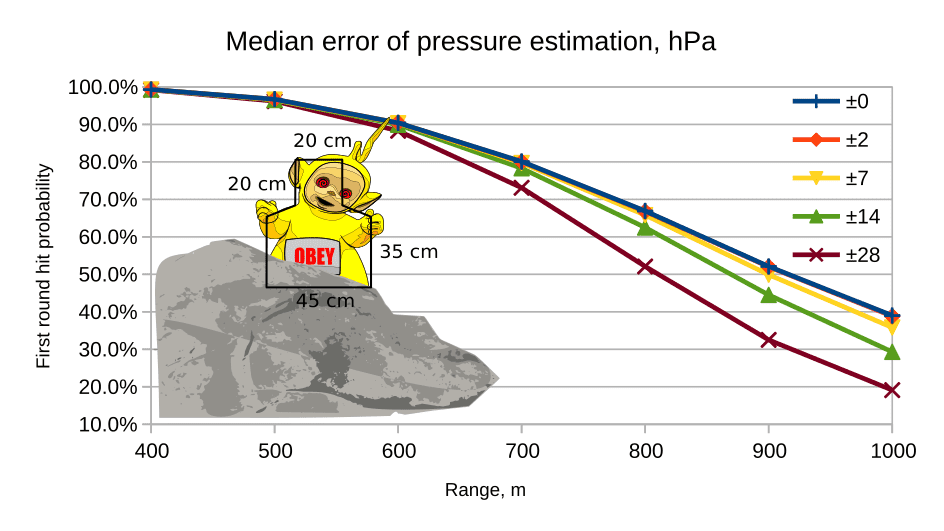
(Lapua Scenar 10 g, cal. 7.62, V0 = 850 m/s, other conditions are assumed excellent)
The scenarios are:
0: ideal
2: Kestrel-style handheld barometer
7: By altitude (std pressure at altitude, ignoring daily and seasonal variations) using map or GPS in mid latitudes of Europe and America. Achtung: this does not apply to deep continental Asia, where seasonal variations are huge.
14: By altitude in Arctic, or anywhere using crappy mechanical pocket barometer
28: Deep continental Asia (e.g. South Siberia) by altitude, or "by feeling" altitude in a mountain relief.
People tend to overestimate the importance of pressure measurements. Most of the time, if you know your altitude and use the standard atmosphere table value, you are very close to the truth (at 1000 m, about 3% loss of hit probability, as compared to the ideal situation). Accurate temperature measurements are WAY more important.
(Lapua Scenar 10 g, cal. 7.62, V0 = 850 m/s, other conditions are assumed excellent)
The scenarios are:
0: ideal
2: Kestrel-style handheld barometer
7: By altitude (std pressure at altitude, ignoring daily and seasonal variations) using map or GPS in mid latitudes of Europe and America. Achtung: this does not apply to deep continental Asia, where seasonal variations are huge.
14: By altitude in Arctic, or anywhere using crappy mechanical pocket barometer
28: Deep continental Asia (e.g. South Siberia) by altitude, or "by feeling" altitude in a mountain relief.
People tend to overestimate the importance of pressure measurements. Most of the time, if you know your altitude and use the standard atmosphere table value, you are very close to the truth (at 1000 m, about 3% loss of hit probability, as compared to the ideal situation). Accurate temperature measurements are WAY more important.
Mikecr
Well-Known Member
LR hunting accuracy is not a game of probabilities.
It's a game of capabilities.
A matter of constant proof with each and every single shot.
It's a game of capabilities.
A matter of constant proof with each and every single shot.