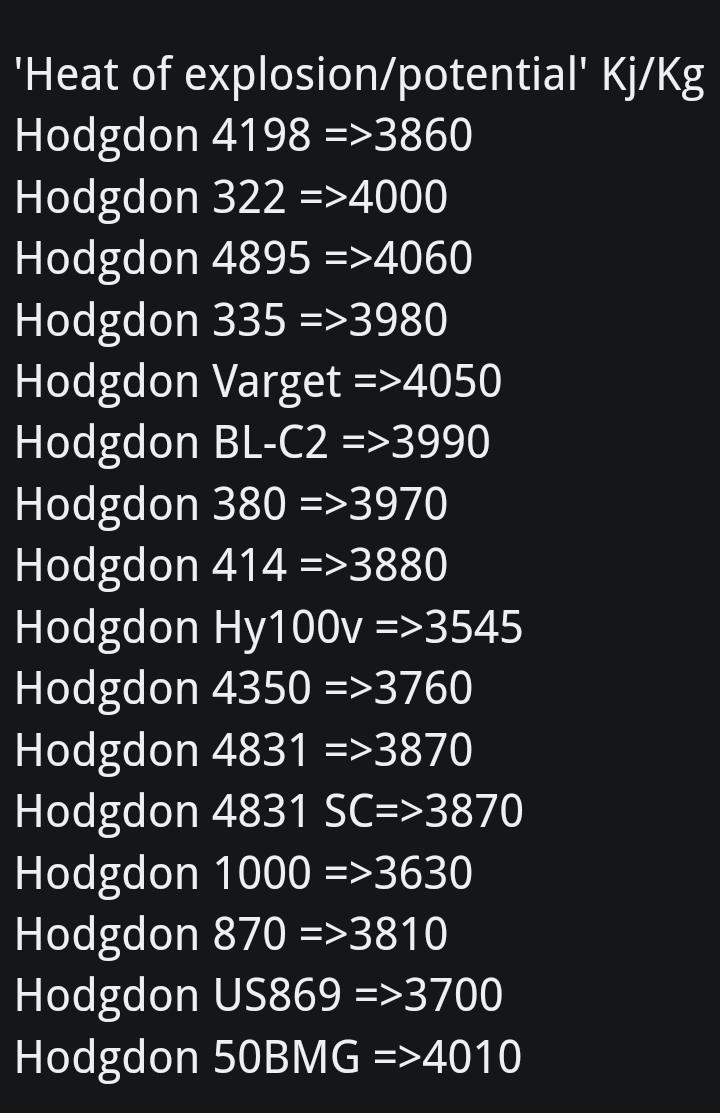
Is there any information online which can explain these relationships in such a way that a person might be able to understand?
As BallisticsGuy alludes to, burn rates are NOT exact between brands, and this is why.
IMR use IMR4895 as their baseline powder to measure RQ numbers. RQ is Relative Quickness. IMR4895 is given a RQ number of #100. All their other powder tested are then either given a higher number (slower burn) or a lower (faster burn) number.
ADI/Thales use AR2206H/H4895 as their #100 RQ baseline, similar to IMR in that respect.
Now, other powder manufacturers such as Alliant/Norma/Vhit/Olin have not made this info public as far as I can ascertain, although the info must be there because QL has got all the relevant info programmed in. I have not been able to source this info even by contacting them.
So, due to powder only EVER being designated a RQ number in house, and no set STANDARD is used to base a RQ number on, there is no way to assume that xxx powder IS actually similar to yyy powder according to a burn rate chart.
BURN RATE IS NOT CONSTANT.
Print this statement out and paste it above your loading bench, and read it before you decide to look at a burn rate chart when choosing a powder to use.
I, and others here, have experienced a powder switching burn rates inexplicably. An example in the 338WM was RE19 behaving much slower than RE22 with 225gr bullets. The RE19 was pushing these bullets over 100fps faster with LESS powder. Have seen the same in the 30-06 with the same 2 powders.
Cheers.